| Uses of Caves | Exploring Caves | References | Timpanogos Cave | Lava Tubes |
How Caves Form
The melt-water streams draining out along the floor of a glacier cave or the surging, pounding waves at the mouth of a sea cave offer immediate evidence of the origin of these caves. Solution caves, however, have always been a source of wonder to man. How do these extensive, complex, and in some places beautifully decorated passageways develop?
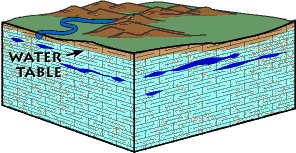 Solution caves are formed in limestone and similar rocks by the action of water; they can be thought of as part of a huge subterranean plumbing system. After a rain, water seeps into cracks and pores of soil and rock and percolates beneath the land surface. Eventually some of the water reaches a zone where all the cracks and pores in the rock are already filled with water. The term water table refers to the upper surface of this saturated zone. calcite (calcium carbonate), the main mineral of limestone, is barely soluble in pure water. Rainwater, however, absorbs some carbon dioxide as it passes through the atmosphere and even more as it drains through soil and decaying vegetation. The water, combining chemically with the carbon dioxide, forms a weak carbonic acid solution. This acid slowly dissolves calcite, forms solution cavities, and excavates passageways. The resulting calcium bicarbonate solution is carried off in the underground drainage system.
Solution caves are formed in limestone and similar rocks by the action of water; they can be thought of as part of a huge subterranean plumbing system. After a rain, water seeps into cracks and pores of soil and rock and percolates beneath the land surface. Eventually some of the water reaches a zone where all the cracks and pores in the rock are already filled with water. The term water table refers to the upper surface of this saturated zone. calcite (calcium carbonate), the main mineral of limestone, is barely soluble in pure water. Rainwater, however, absorbs some carbon dioxide as it passes through the atmosphere and even more as it drains through soil and decaying vegetation. The water, combining chemically with the carbon dioxide, forms a weak carbonic acid solution. This acid slowly dissolves calcite, forms solution cavities, and excavates passageways. The resulting calcium bicarbonate solution is carried off in the underground drainage system.
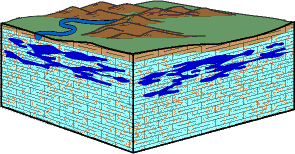 It was once believed that caves formed near the Earth's surface-above the saturated zone-where the water moved downward through the cracks and pore spaces. This view, however, left many cave features unexplained.
It was once believed that caves formed near the Earth's surface-above the saturated zone-where the water moved downward through the cracks and pore spaces. This view, however, left many cave features unexplained.
Why, for instance, are cave passages nearly horizontal, in places crossing folded or tilted rock structures? How would horizontal passages form at several different but persistent levels? Recent studies of the movement and chemistry of ground water have shown that the first stage in cave development-the dissolving of carbonate rocks and the formation of cavities and passage-ways-takes place principally just below the water table in the zone of saturation where continuous mass movement of water occurs.
A second stage in cave development occurs after a lowering of the water table (the water table normally sinks as the river valleys deepen). During this stage, the solution cavities are stranded in the unsaturated zone where air can enter. This leads to the deposition of calcite, which forms a wide variety of dripstone features.
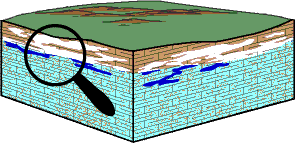 The chemical process causing deposition of calcite is the reverse of the process of solution. Water in the unsaturated zone, which dissolved some calcite as it trickled down through the limestone above the cave, is still enriched with carbon dioxide when it reaches the ventilated cave. The carbon dioxide gas escapes from the water (just as it escapes from an opened bottle of soda pop). The acidity of the water is thereby reduced, the calcium bicarbonate cannot remain in solution, and calcite is deposited as dripstone.
The chemical process causing deposition of calcite is the reverse of the process of solution. Water in the unsaturated zone, which dissolved some calcite as it trickled down through the limestone above the cave, is still enriched with carbon dioxide when it reaches the ventilated cave. The carbon dioxide gas escapes from the water (just as it escapes from an opened bottle of soda pop). The acidity of the water is thereby reduced, the calcium bicarbonate cannot remain in solution, and calcite is deposited as dripstone.